 A Critically Swift Response: Insulin-Stimulated Potassium and Glucose Transport in Skeletal Muscle | American Society of Nephrology
A Critically Swift Response: Insulin-Stimulated Potassium and Glucose Transport in Skeletal Muscle | American Society of NephrologyWarning: The NCBI website requires JavaScript to operate. Insulin for the treatment of hypercalemia: a double-edged sword? Potassium plays a critical role in cell metabolism and normal neuromuscular function. In the process of evolution, strictly regulated homeostatic mechanisms have been developed to provide primary defense against hyper- and hypokalemia threats. The kidney plays a primary role in potassium balance, increasing or decreasing the rate of potassium excretion. Potassium distribution between intracellular compartments and extracellular fluids is regulated by physiological factors such as insulin and catechins that stimulate the activity of ATPase Na+-K+. Only about 10% of the ingested potassium is excreted through the intestine under normal physiological conditions []. Patients with end-stage kidney disease (RED) rely heavily on extrarenal mechanisms and dialysis to maintain potassium homeostasis. Despite the availability of dialysis and the adaptive increase in potassium excretion in renal insufficiency, severe hyperkalemia (defined as the level of serum potassium 6 mEq/L [6 mmol/L]) is observed in 5-10% of patients with maintenance dialysis and is responsible for 0.7 per cent of deaths in the dialysis population in the United States [–]. Several factors can explain the high incidence of hyperkalemia in this population. Tolerance for a rapid potassium load is affected in ESRD, not only by lack of renal excretion, but also by poor cell distribution of potassium []. The latter may result from defect in the ATPase Na+-K+ and possibly high levels of glucagon in uremia [, ]. Intake of high diet potassium and lost dialysis treatments are common contributors to hyperkalemia in SRD patients. Other factors such as constipation (reduced colonized excretion) and fasting status (relative insulin) may also predispose patients with SRD to hyperkalemia []. Patients who are new to dialysis may have some residual kidney function and remain sensitive to several kinds of medications that damage potassium excretion. These include renin-angiotensin-aldosterone (RAAS), potassium spacing diuretics, heparin, trimethoprima, pentamidine, and nonsteroidal anti-inflammatory drugs (NSAIDS). The use of spironolactone in patients with prevalent SRD has also been involved as a cause of hyperkalemia due to its effect on colonic potassium management. Non-selective beta blockers can also lead to hyperkalemia by avoiding intracellular changes in potassium []. The treatment of acute hyperkalemia in patients with SRD is emerging dialysis. When dialysis is not immediately available, temporary measures are used. Intravenous calcium is used to counteract the adverse myocardial effects of hyperkalemia, but does not affect the serum concentration of potassium. cation exchange resins are frequently used to increase potassium secretion. However, due to its late appearance of action (at least two hours), questionable efficacy and possible toxicities such as chronic necrosis, cation exchange resins are not recommended first-line therapy in acute hyperkalemia []. The use of intravenous bicarbonate therapy has also been discouraged due to lack of benefit [, ]. Other measures that result in the change of potassium from extracellular to intracellular space, such as albuterol and insulin, have proven to be effective in patients with chronic kidney disease (CKD) and are faster at the beginning, usually more than 30-60 minutes [, –]. However, for unclear reasons, a subset of ESRD patients is resistant to albuterol actions []. Intravenous insulin (IV) is therefore the first-line therapy for acute hyperkalemia in patients hospitalized with DRD. It is usually used in conjunction with dextrose to prevent hypoglycemia, and is often combined with other therapies such as nebulized albuterol. Although insulin-mediated glucose absorption is affected in the uremia, the effect of insulin potassium attenuation is not affected []. It is believed that this is due to independent pathways for the transport of potassium and glucose through the cell membrane []. Insulin changes potassium in cells by stimulating the activity of the Na+-H+ antipertoire on the cell membrane, promoting the entry of sodium into the cells, leading to the activation of the Na+-K+ ATPase, causing an electrogenic flow of potassium. Insulin IV leads to a dose-dependent decrease in serum potassium levels []. A combination of insulin IV doses of 10 units plus 25 g of dextrose reliably reduces the level of serum potassium by 1 mEq/L (mmol/L) in 10-20 minutes and the effect lasts about 4-6 hours [, ]. However, this therapy may be associated with significant hypoglyemia [, –]. In a study of patients hospitalized treated with intravenous insulin by hyperkalemia, 8.7 per cent of patients developed hypoglycemia (defined as blood glucose of the definition of hypoglycemia has been a topic of debate. The working group of the American Diabetes Association and the Endocrine Society defines iatrogenic hypoglycemia in patients with diabetes mellitus (DM) as "all episodes of an abnormally low plasma glucose concentration that expose the individual to potential damage." A plasma glucose concentration of ≤70 mg/dL [3.9 mmol/L] is recommended as an alert value, although symptoms of hypoglycemia are usually developed at a lower level of this threshold [, ]. This value allows time for close patient follow-up to prevent symptomatic hypoglycemia and has been used to define hypoglycemia in numerous clinical trials. Hypoglycemia in the hospital environment is a common problem, with incidences ranging from 3.5% to 10.5% in general medications, 23% in surgical patients and 10% in ICU []. Symptoms of hypoglycemia may include irritability, impaired cognitive function, seizures, coma, ventricular arrhythmias and even death [, , ]. There are multiple risk factors for hypoglycemia. These include medications, drug interaction, endogenous insulin deficiency (respons to concomitant abnormal glucago), critical disease, poor nutritional intake, low body weight, older age, history of recurrent hypoglycemia, liver failure, and kidney failure [, , ].In this number of clinical units in Kidney, Apel et al [] report the incidence and time of hypoglycemia, In this uncontrolled retrospective study, Apel et al found an incidence of 13% hypoglycemia (defined as blood glucose) Patients with CKD and ESRD are particularly at risk of hypoglycemia [, ]. In the establishment of normal kidney function, the kidney contributes to almost half of general gluconeogenesis, and therefore it is as important as the liver in carbohydrate metabolism [, ]. The kidney also plays a critical role in insulin metabolism. Although the evidence implies that CDK creates an insulin-resistant state, hypoglycemia can occur due to decreased gluconeogenesis and insulin degradation []. Other factors such as altered drug metabolism, malnutrition, hepatic gluconeogenesis decreased, and the infection also increases the risk of hypoglycemia in this population [, ]. In patients with hemodialysis, the use of glucose-free dialysis solution increases the risk of hypoglycemia due to the transfer of plasma glucose to dialysis. The addition of glucose to dialysis solution significantly decreases this risk [, ]. Apel et al do not specify in their study if there is a difference between patients of hemodialysis and peritoneal dialysis. Theoretically, the incidence of hypoglycemia is lower in peritoneal dialysis due to the presence of dextrosis in the dialysis solution. The results of the Apel study emphasize the importance of intense blood glucose monitoring after insulin administration. The Apel study defined hypoglycemia as ≤60 mg/dL [3.33 mmol/L] and even with this conservative definition, the incidence was 13%. Since insulin IV is a therapy commonly used for severe hyperkalemia in patients with ESRD in the hospital environment, we agree with Apel et that a protocol-based approach may be able to reduce the incidence of hypoglycemia. The published literature indicates that the insulin and dextrose regime varies from center to center. The insulin dose varies from 5 to 10 units and the amount of glucose varies from 25 to 60 g []. Others have recommended additional infusion of dextrose after intravenous push of dextrose and insulin to prevent hypoglycemia [, ]. Due to the risks of hypoglycemia, some have advocated the use of glucose alone in the treatment of hyperkalemia. Rationality is based on the theory that exogenous glucose stimulates the secretion of insulin that moves potassium to the cell. In a randomized and cross-sectional study of 10 non-diabetic patients, ESRD in hemodialysis with hyperkalemia, dextrose alone led to a clinically significant decrease in the level of serum potassium. Hyperglycemia rates were not reported []. However, some concerns have been raised regarding this approach. Endogenous insulin secretion can be unpredictable, especially in acute patients and those with insulin deficiency [, ]. The resulting hyperglycemia elevates the plasma osmolality, which leads to the movement of potassium outside the cell, making hyperkalemia worse. On the contrary, some have suggested the use of insulin alone in the establishment of hyperglycemia, but this is not widely accepted or practiced due to the high probability of inducing hypoglycemia [, ].Similar to the current study, Shafers et al also observed that the majority (74%) of patients who developed hypoglycemia did not have a diagnosis of DM at the time of treatment for hyperkalemia. This is an important point as patients without DM are at risk of lack of monitoring their blood glucose levels. Hospital staff are trained to monitor blood glucose in patients with DM and the absence of this diagnosis makes the patient more vulnerable. In addition, patients without DM have greater insulin sensitivity and are more likely to develop hypoglycemia after insulin administration. The protocol proposed by Apel et al in this study for glucose monitoring and dextrose support in the treatment of insulin IV hyperkalemia is designed to prevent hypoglycemia. We agree that the risk of hypoglycemia can be minimized by increasing the dose of dextrose. However, there may be a risk of hyperglycemia if 25 g of dextrose is given 1 hour after the administration of insulin/dextrose "respective of the blood glucose level". Based on the experience in our institution and the recommendations of experts, we have that a blood glucose level of ≥250 mg/d L will not require additional doses of intravenous dextrose, after the initial 25 g. However, this recommendation has not been validated in clinical trials. In our center, the body weight of the patient is taken into account before administering insulin and dextrose. The protocol in our center is to administer 25 g dextrosis with insulin IV 0.1 units/kg body weight. This regime is followed by 250 mL of D10W infused more than 2 hours. The use of a weight-based insulin regimen reduces the risk of hypoglyemia in individuals with low body mass index, especially in elderly people. Limited data have suggested that the administration of dextrosis before insulin is effective and safe []. In our center, dextrose is given immediately before insulin IV. Blood glucose levels are obtained at the basic level, 1, 2 and 3 hours after treatment. Since the implementation of this protocol, the incidence of hypoglycemia in patients hospitalized in our center has increased from approximately 20% to less than 5%. As mentioned above, beta-agonists, such as inhaled albuterol, have an additive effect of potassium drop due to a different mechanism of action. Beta-agonists, when used with insulin, may have the additional benefit of reducing the risk of hypoglycemia as they promote gluconeogenesis in the liver [, ].In conclusion, patients with ESRD are at high risk of developing severe and life-threatening hyperkalemia. When dialysis is not immediately available, non-diagnostic therapies are used as tempting measures. Insulin treatment is effective, but may be associated with severe hypoglycemia if appropriate therapeutic guidelines are not applied and practiced. The education of doctors and nursing staff, and the adherence to a treatment algorithm specific to hyperkalemia are extremely important to prevent this critical iatrogenic complication. Disclosures. None declared. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
What is the connection between diabetes and potassium? Is there a link? Usually, your body processes the food you eat and makes it a sugar called glucose. Your body uses glucose for energy. Insulin is a hormone that produces. Your body uses insulin to help move glucose into cells through your body. If you have , your body is unable to produce or use insulin efficiently. is not preventable, but you can prevent. Type 2 diabetes, or adult diabetes, usually occurs in people over 35 years of age. Potassium is an electrolyte and a mineral that helps keep your body fluids at the right level. Your body can do the following if your fluids are in control: If you do not maintain the right potassium level, you may experience a variety of symptoms that include simple muscle cramps to more severe conditions, such as seizures. According to recent research, there may be a link between type 2 diabetes and low potassium levels. Although people recognize that potassium affects diabetes, research is ongoing to determine why this can happen. Researchers at one of the Johns Hopkins University School of Medicine linked low potassium levels to high levels of insulin and glucose in people who were otherwise healthy. Low potassium levels with high levels of insulin and glucose are both traits that doctors associate with diabetes. A 2011 study found that people who take tiazide to treat high blood pressure experienced a loss of electrolytes, such as potassium. Researchers noted that this loss could increase a person's risk of developing diabetes. And along with that, researchers have also linked potassium levels to high blood pressure. Although low potassium may increase your risk of developing diabetes, taking potassium will not cure your diabetes. On average, people over 14 years of age should consume about 4,700 milligrams, or 4.7 grams, potassium per day. Even if you are getting as much potassium as you need, your levels can still be too high or low. This can happen for several reasons, including a change in your sodium levels. When sodium levels increase, potassium levels tend to fall and vice versa. Other possibilities include: Certain anti-diabetes medicines may affect your potassium levels. For example, if you take insulin and have not maintained control of your diabetes, your potassium levels can decrease. If you think you are at risk for diabetes, or that you may have a potassium deficiency, make an appointment with your doctor. You can review your medical history and discuss your potential risk. Your doctor can see how much potassium is in your blood doing a blood test. If the test shows that your potassium levels are abnormal, your doctor may prescribe a supplement or recommend certain dietary changes to restore balance. You should strive to consume 4.7 grams of potassium every day to keep your potassium in control. You can do this by monitoring your daily intake using a food journal and actively investigating how much potassium is in the food you eat. Some of the best potassium sources are: You should limit your consumption of processed foods because they are a poor source of potassium. If you work regularly and sweat a lot, consider adding a post-workout banana smoothie to your routine. This can replenish some of the potassium you lost and help balance the electrolyte levels of your body. If you feel you are not getting enough potassium, make an appointment with your doctor. They can work with you to develop the best course of action. With some advanced monitoring and planning on your diet, you can control your potassium levels and help prevent diabetes. Last medical review on July 26, 2017Read this following

Physiologic Effects of Insulin
Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference - Kidney International
Insulin and Potassium | Diabetes Library
Disorders of Potassium Balance: Hypokalemia & Hyperkalemia | Abdominal Key
Physiologic Effects of Insulin
Glucose-Insulin-Potassium Revived | Circulation
Management of severe hyperkalemia in the post-Kayexalate era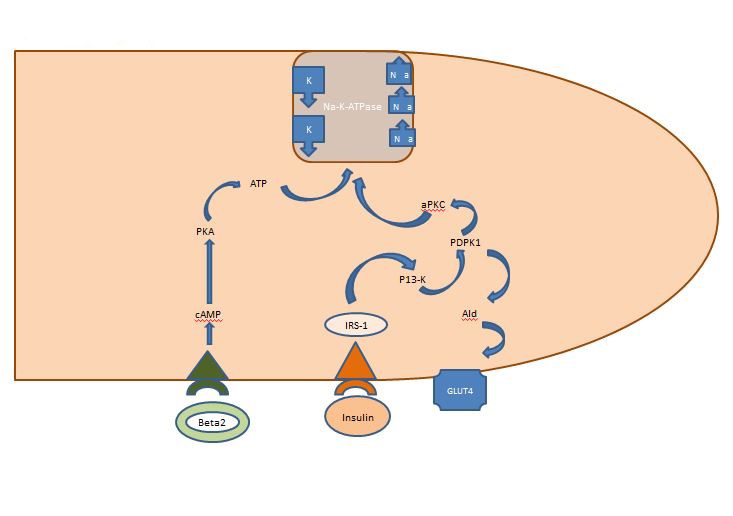
First in a series on hyperkalemia: hyperkalemia, the sodium potassium pump and the heart
Ashcroft Group — Department of Physiology, Anatomy and Genetics (DPAG)
Regulation of Potassium Homeostasis | American Society of Nephrology
Insulin - Wikipedia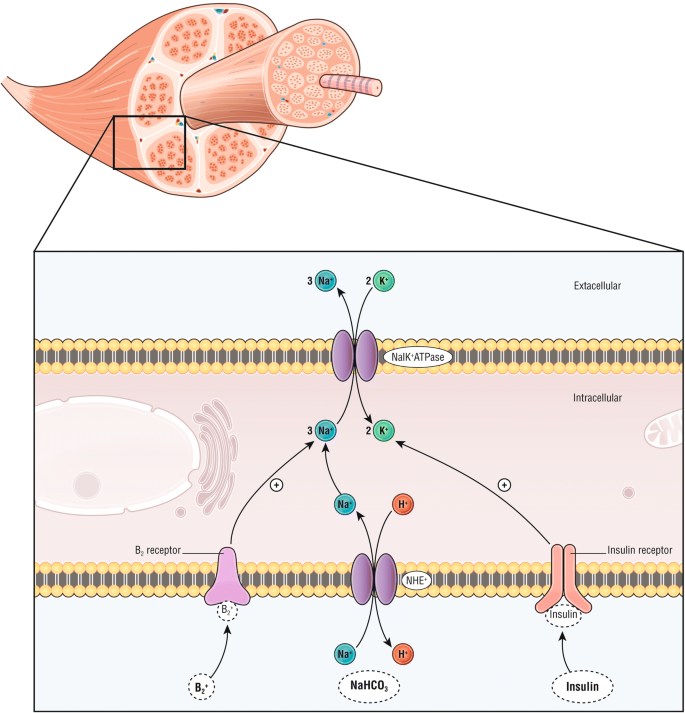
Management of hyperkalemia in the acutely ill patient | Annals of Intensive Care | Full Text
Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019 - American Journal of Kidney Diseases
Glucose-Insulin-Potassium Revived | Circulation
KATP channels regulate insulin secretion in pancreatic β-cells. Under... | Download Scientific Diagram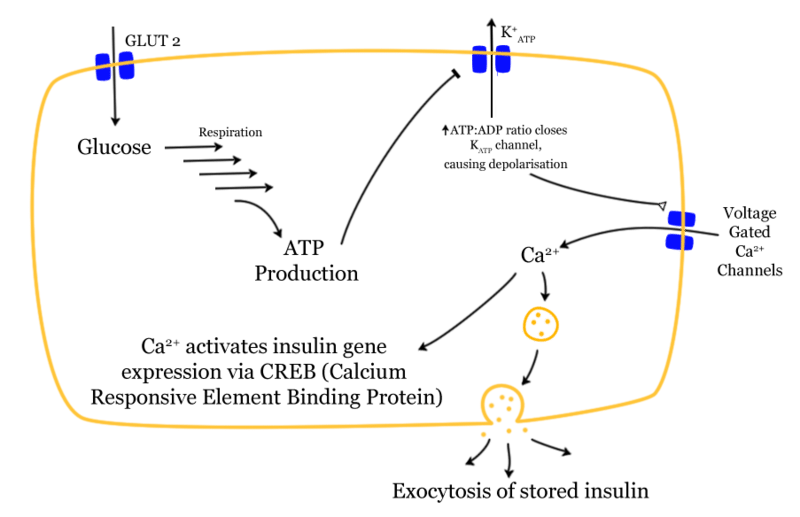
Insulin - Structure - Function - TeachMePhysiology
Hyperkalemia - American Family Physician
Glucagon actions on the kidney revisited: possible role in potassium homeostasis | American Journal of Physiology-Renal Physiology
Diabetes and Insulin Secretion | Diabetes
Potassium Homeostasis, Chronic Kidney Disease, and the Plant-Enriched Diets | American Society of Nephrology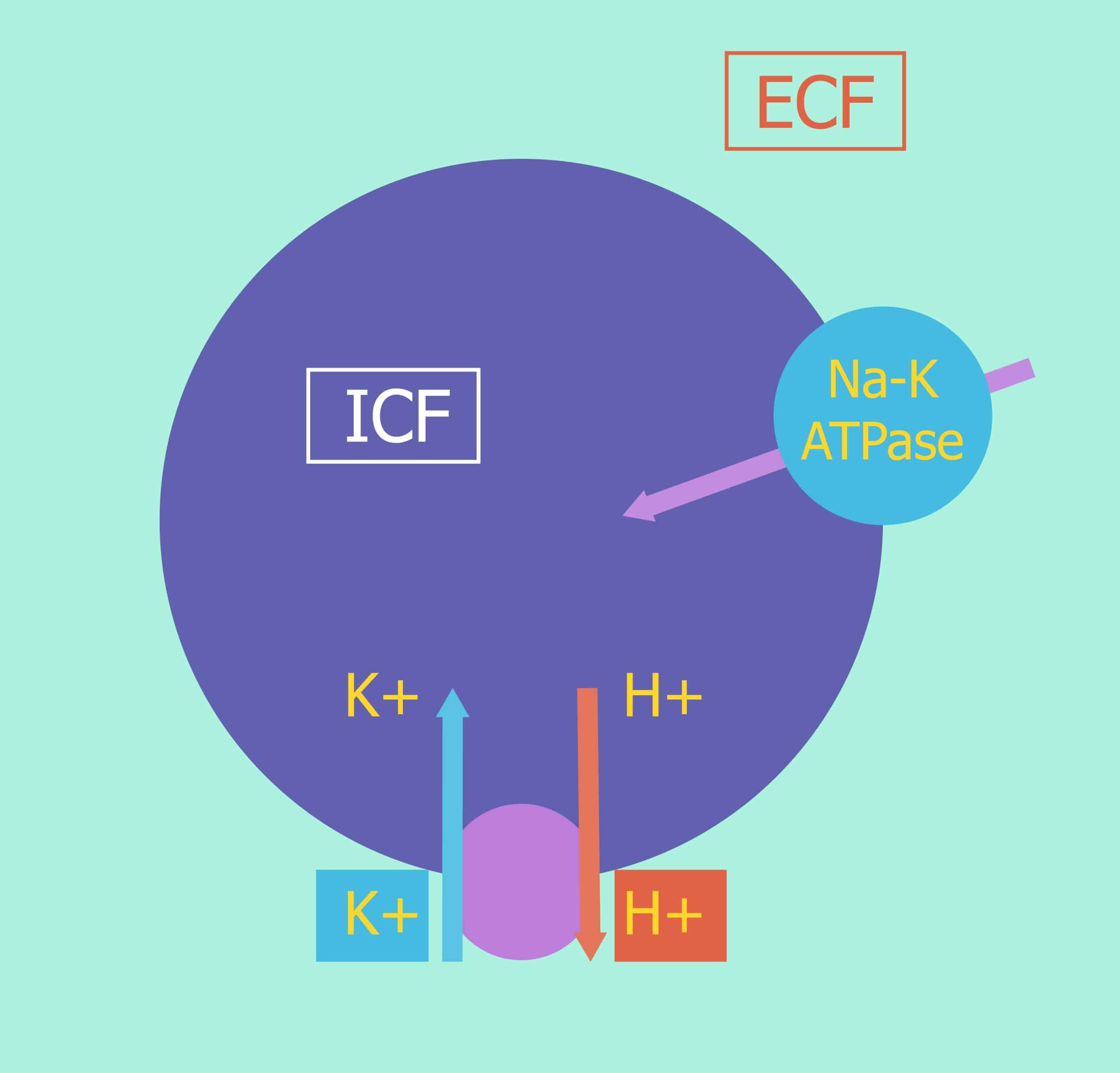
Internal Balance of Potassium - Moving Into Cells - TeachMePhysiology
Hypokalemia: a clinical update in: Endocrine Connections Volume 7 Issue 4 (2018)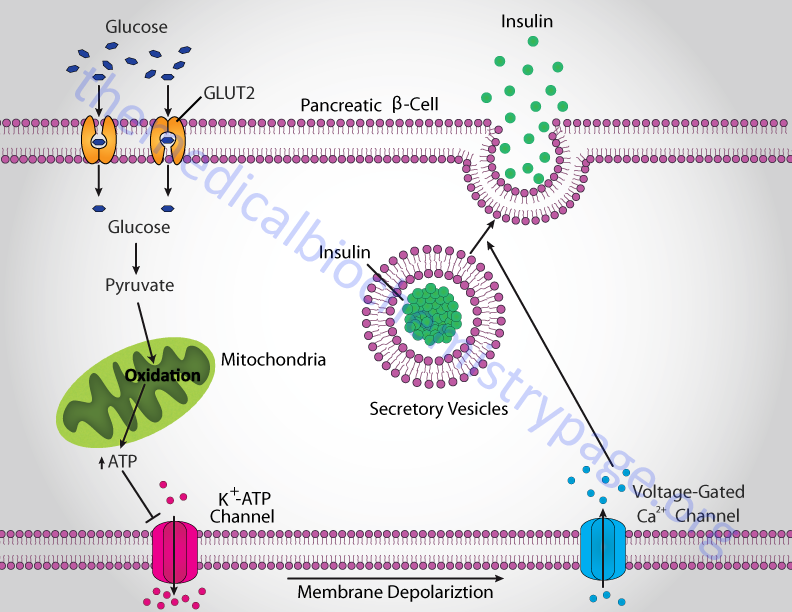
Insulin Function, Insulin Resistance, and Food Intake Control of Secretion - The Medical Biochemistry Page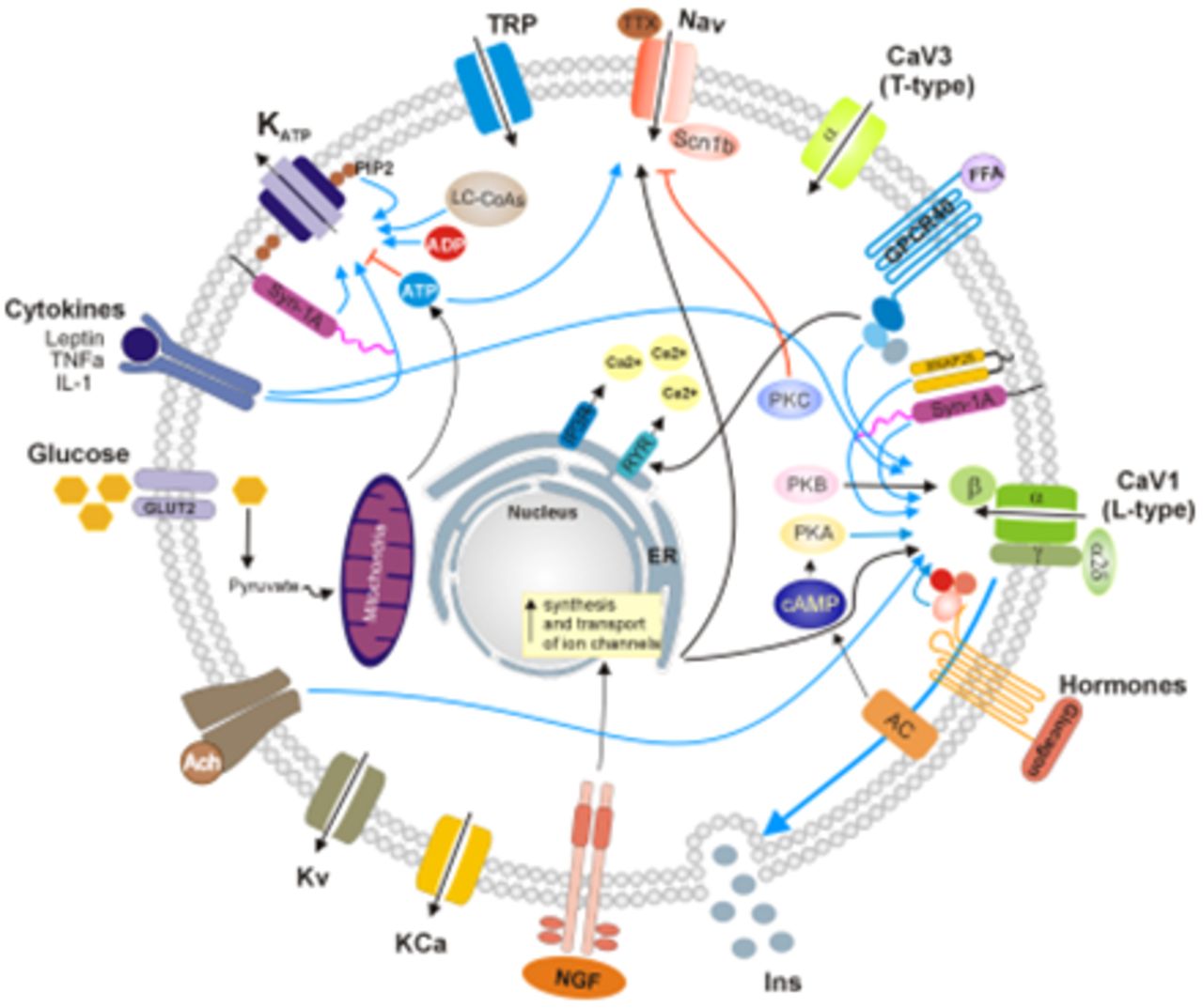
Modulation of Ionic Channels and Insulin Secretion by Drugs and Hormones in Pancreatic Beta Cells | Molecular Pharmacology
Insulin - Wikipedia
Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? | Diabetes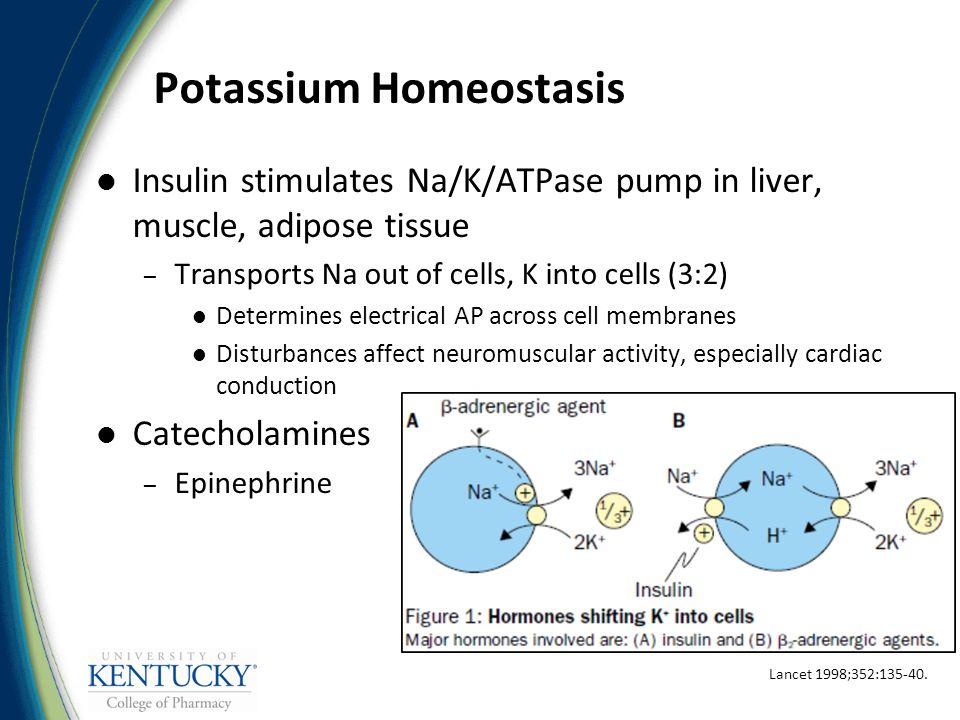
Disorders of Electrolyte Homeostasis – Potassium and Magnesium - ppt video online download
Hyperkalaemia | The BMJ
Hypokalemia - YouTube
Management of severe hyperkalemia in the post-Kayexalate era
Effects of pH on Potassium: New Explanations for Old Observations | American Society of Nephrology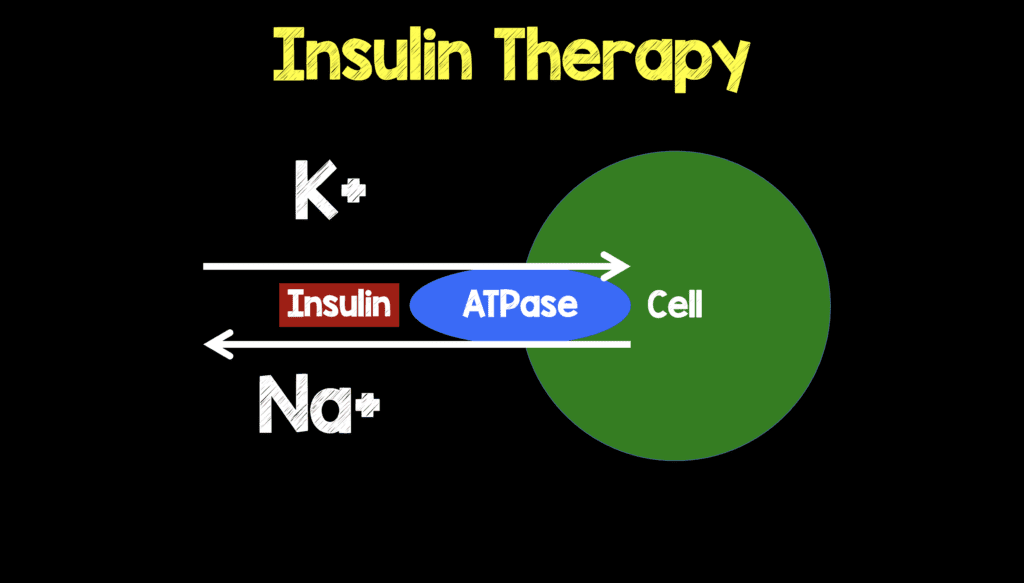
Mythbuster – REBEL EM – Emergency Medicine Blog
Why doesn't regular insulin therapy cause hypokalemia in patients with diabetes mellitus? - Quora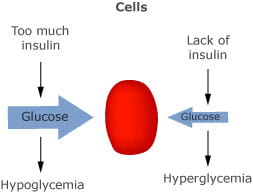
Diabetic ketoacidosis
Hyperkalemia pathophysiology - wikidoc
Congenital Hyperinsulinism - an overview | ScienceDirect Topics
emDOCs.net – Emergency Medicine EducationInsulin Dosing in Hyperkalemia – Is It a One Size Fits All? - emDOCs.net - Emergency Medicine Education
How Does Insulin Affect Potassium Levels?
Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019 - American Journal of Kidney Diseases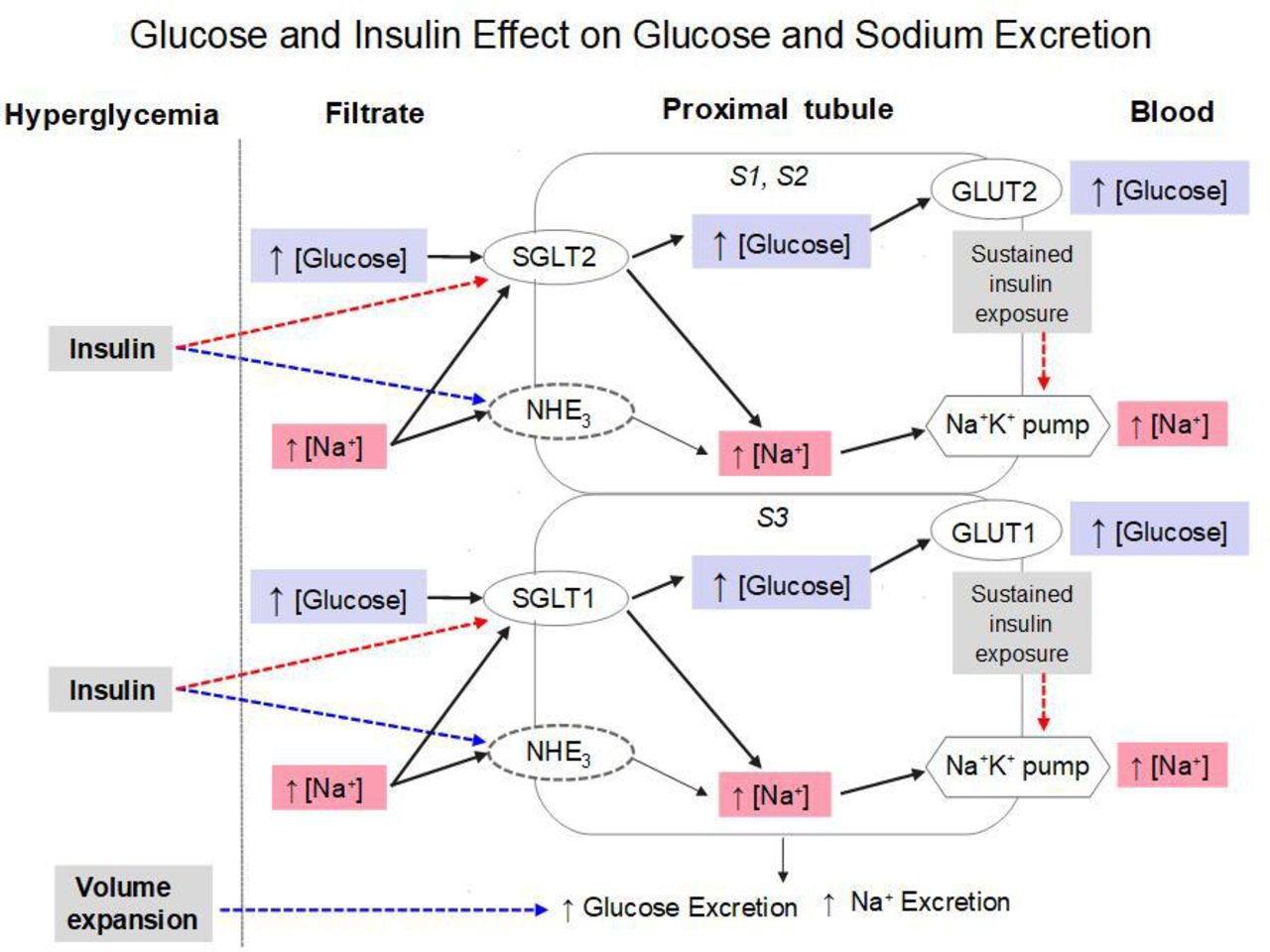
Insulin enhances renal glucose excretion: relation to insulin sensitivity and sodium-glucose cotransport | BMJ Open Diabetes Research & Care
 A Critically Swift Response: Insulin-Stimulated Potassium and Glucose Transport in Skeletal Muscle | American Society of Nephrology
A Critically Swift Response: Insulin-Stimulated Potassium and Glucose Transport in Skeletal Muscle | American Society of Nephrology




































Posting Komentar untuk "how does insulin affect potassium"